cross-posted from: https://sh.itjust.works/post/31544365
Reduced glucose metabolism and mitochondrial dysfunction correlate with increased neuronal death and brain damage during cerebral ischemia and neurodegeneration. Ketone bodies (KBs), such as acetoacetate and β-hydroxybutyrate, serve as crucial alternative energy sources during glucose deficiency. Both KBs and the ketogenic diet (KD) demonstrate neuroprotective effects by orchestrating various cellular processes through metabolic and signaling functions.
Thank you @HylicManoeuvre@sh.itjust.works for sharing the paper its really interesting!
This is a really great overview paper, from 2024. If your interested in this subject I recommend reading the full paper.
Notes:
In adults, plasma KB concentrations typically range from 0.05–0.1 mM under normal conditions, rising to 1–2 mM after 2 days of fasting and reaching 5–8 mM during prolonged fasting and starvation
While glucose is the primary energy source under normal conditions, the brain adapts to KBs during starvation and glucose deprivation.
The author is showing bias in their assumptions by the term normal; It would be better if they indicated what the preferred brain fuel was/is (ketones or glucose) rather then saying “normal”, because that implies bias.
Although diabetic ketoacidosis is a pathological condition, mild ketonemia resulting from caloric restriction (CR), fasting, vigorous exercise, and KD, has proven beneficial and extends a healthy lifespan
I’m not sure why they are talking about ketoacidosis at all, is it relevant?
figure 1 - ketogenic and ketolytic pathways in the liver and braincells
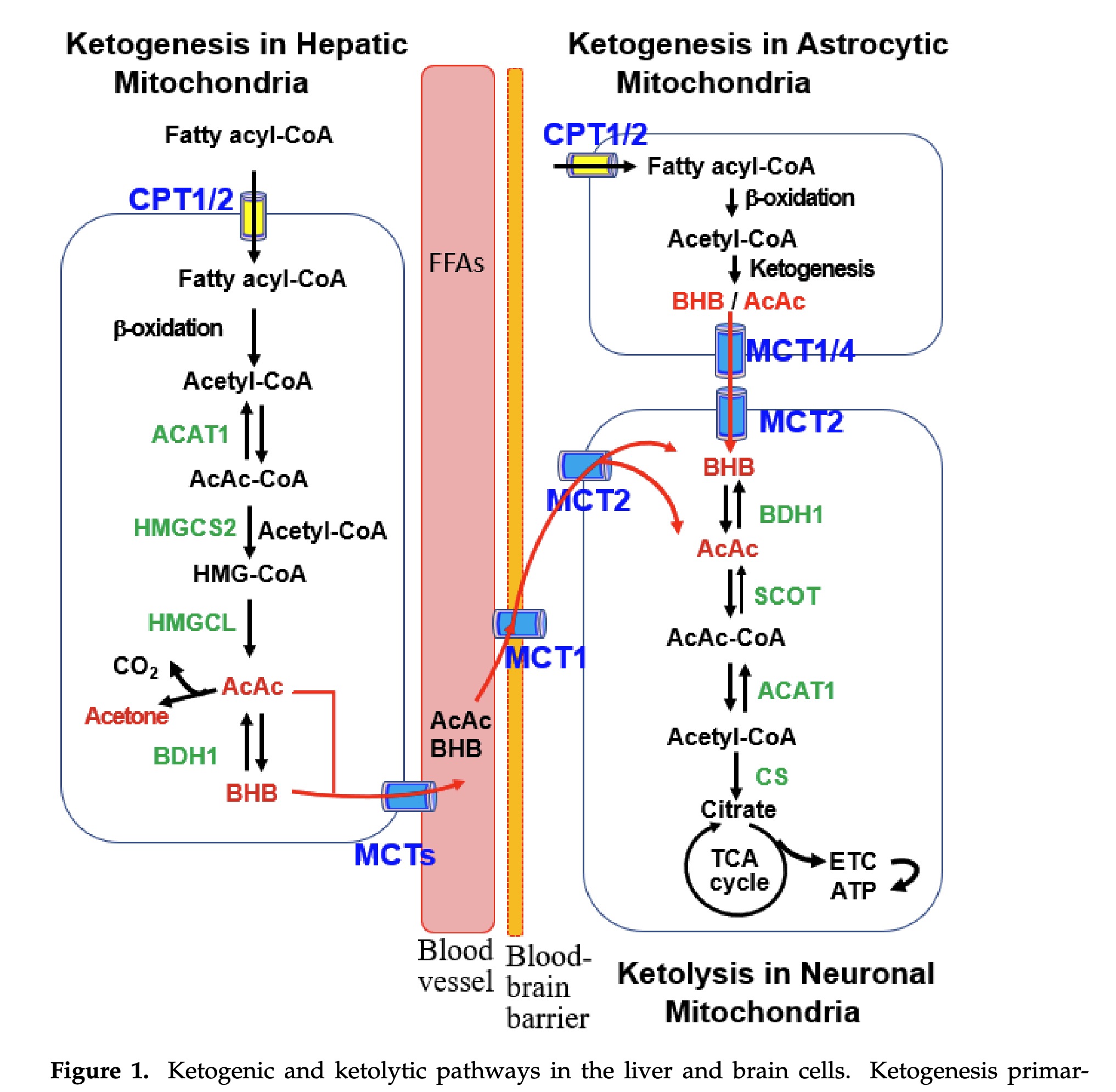
Liver-derived blood KBs, including BHB and AcAc, traverse the blood-brain barrier (BBB) to reach the brain through facilitated diffusion mediated by MCTs
It’s important to note this transversal is not impacted by insulin resistance. They hint at this when talking about AD but do not say it explicitly.
The extent of KB uptake and ketolysis in the brain is largely influenced by blood KB concentrations
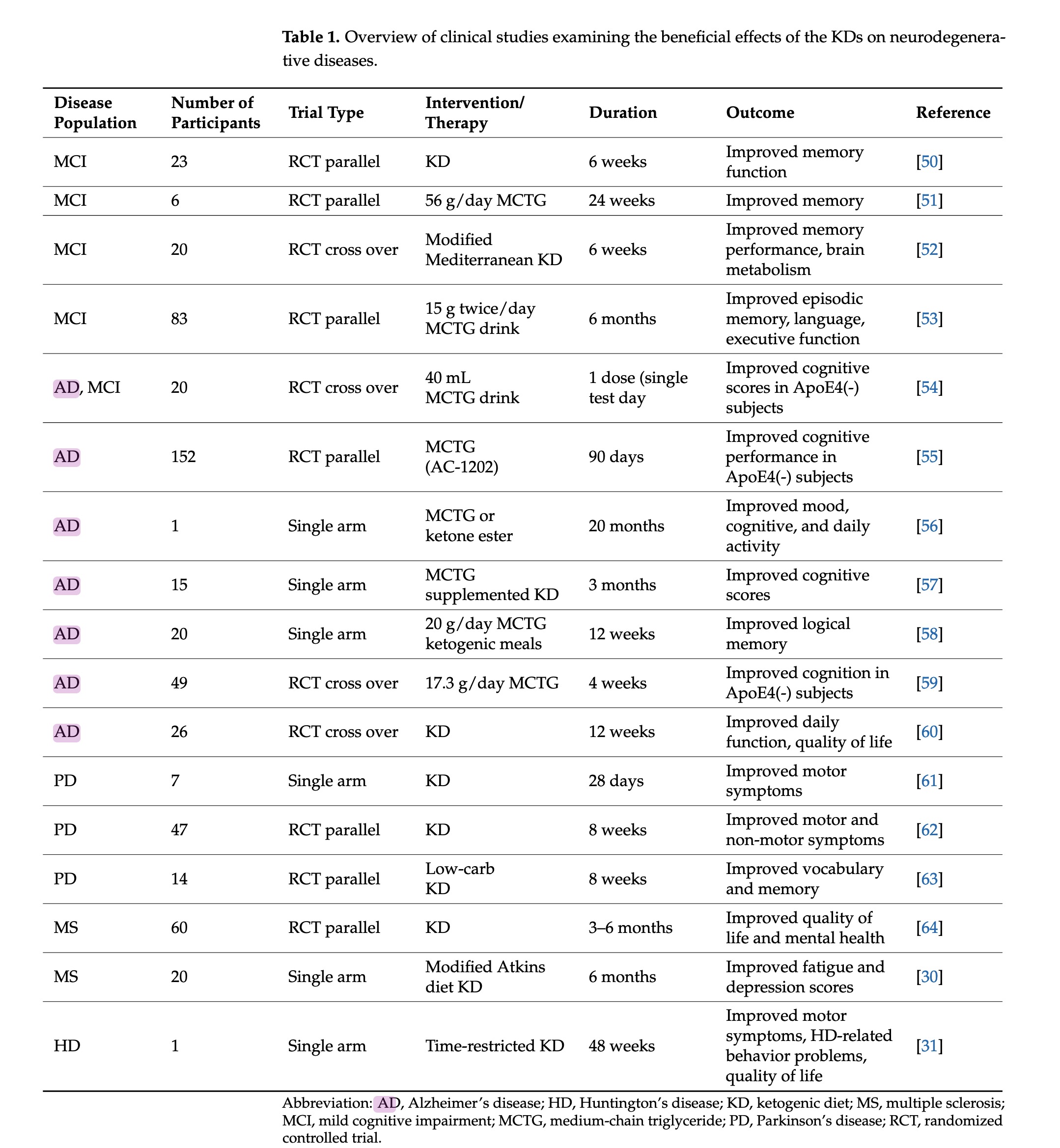
table 1 - overview of clinical studies using ketones
Consumption of a KD 3 days prior to stroke induction improves mobility in endothelin-1 model rats, indicating the benefit of KD preconditioning…Moreover, pre-injury KD consumption prevented neurodegeneration due to cardiac arrest-induced cerebral ischemia
Although the causes of AD are not yet fully understood, it is proposed that a combination of harmful factors such as age-related changes in the brain, oxidative stress, inflammation, blood vessel damage, and lack of energy supply to the brain are involved.
This strongly implies a link to insulin resistance, and the inability of the glucose/insulin to get into the brain at the proper ratios.
These preclinical and clinical observations suggest that KBs and KD may have therapeutic potential in the treatment of PD.
In contrast to glucose, the utilization of KBs remains unaffected in the brains of elderly individuals and patients with AD.
figure 2 - neuroprotective effects of KBs and KD .... in the brain
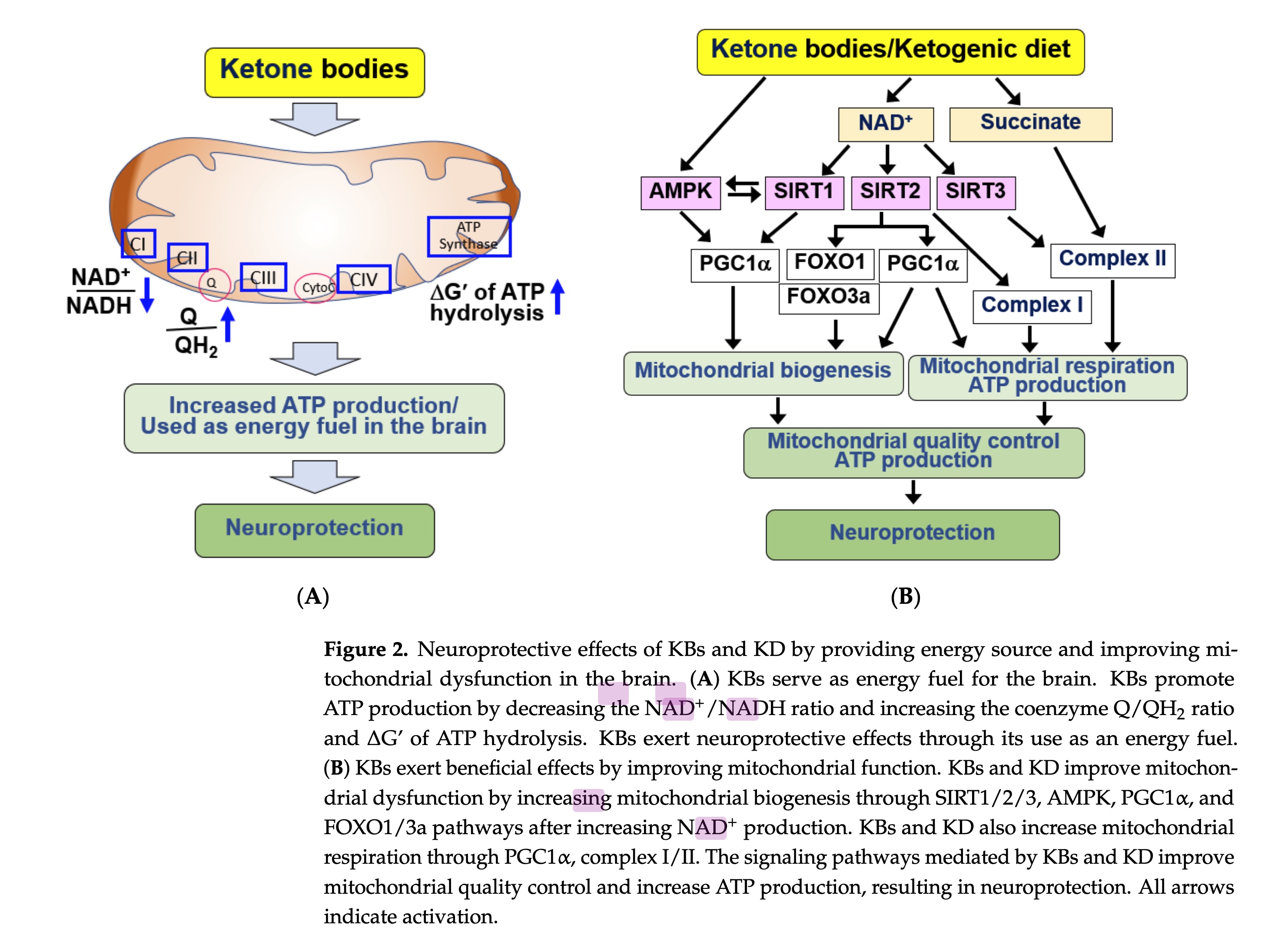
The molecular mechanism underlying the beneficial effects of KBs and KD involves increased NADH oxidation via KB catabolism [ 39, 82]. Elevated NAD+ levels play crucial roles, such as stimulating mitochondrial biogenesis and respiration, by serving as a cofactor for NAD±dependent enzymes in cellular signaling processes
the neuroprotective effect of BHB is linked to the improvement of mitochondrial complexes I and II and the resulting enhancement of ATP production [ 69]. Similar to KBs, KD-induced nutritional ketosis increases both mitochondrial respiration and homeostasis, suggesting its therapeutic potential for the treatment of chronic and degenerative diseases with mitochondrial dysfunction
figure 3 - signaling functions of KBs and KD

finding indicates that BHB may extend lifespan and prevent age-related neurodegeneration through HDAC inhibition
figure 4 - signaling KD,KB for anti-inflammation and autophagic flux
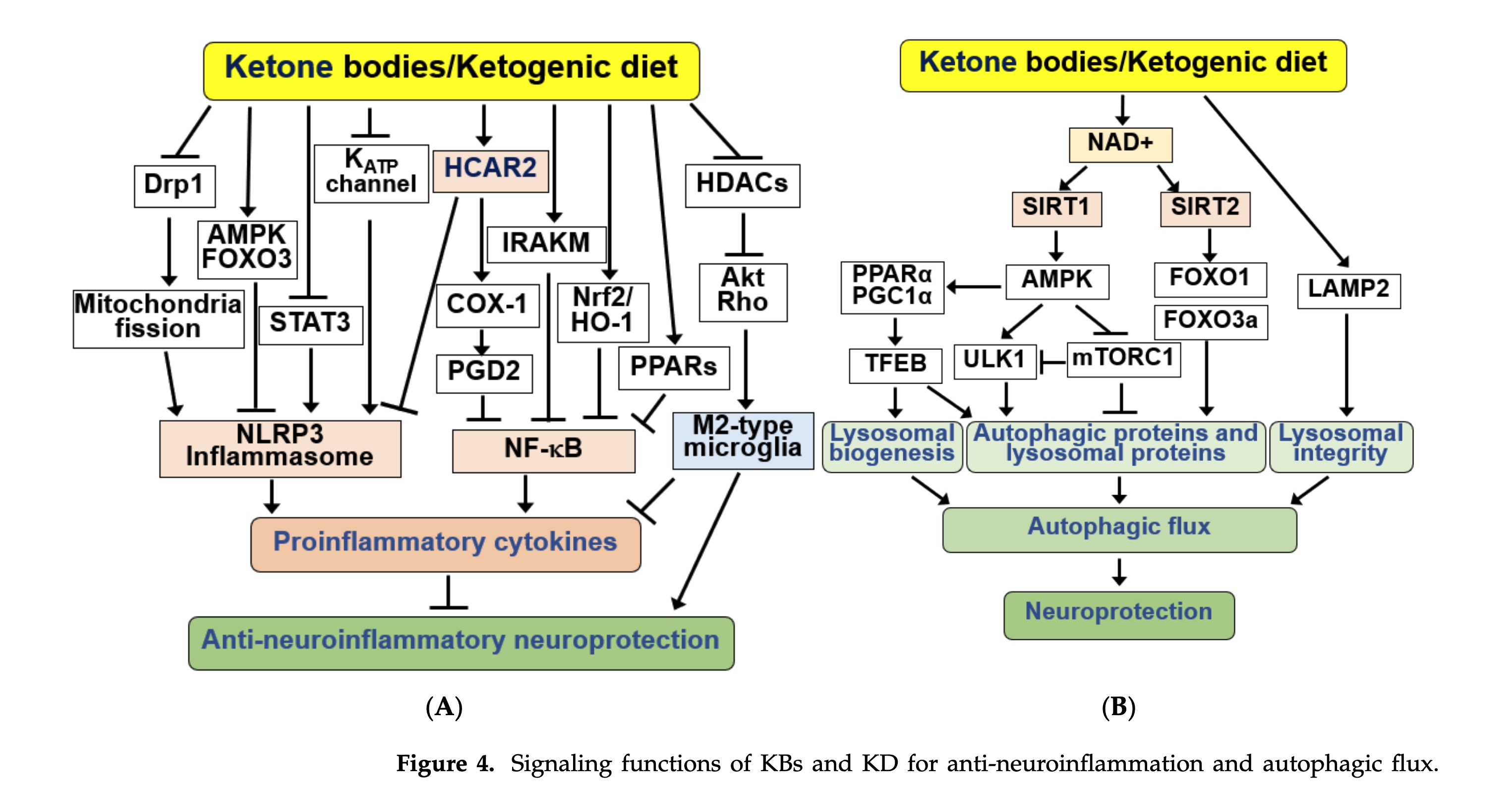
4.6. KBs and KD as Regulators of Autophagy
Autophagy, a lysosome-dependent degradation process, is a key mechanism in maintaining cellular homeostasis and survival. Impaired autophagy in the brain results in the accumulation of abnormal protein aggregates and neuronal loss, culminating in neurodegenerative disorders [182]. Therefore, maintaining proper autophagy is essential to prevent accelerated neurodegeneration
suggesting a potential interconnection between KB metabolism and autophagy
Collectively, the neuroprotective effects of KBs and KD appear to be linked to their ability to stimulate autophagic flux and correct autophagy defects
In cultured neurons, the replacement of glucose with BHB leads to changes in metabolic pathways, enhancing the TCA cycle flux, producing more α-ketoglutarate, and ultimately converting to GABA in GABAergic neurons
figure 5 - regulation of neurotransmission and gut microbiota
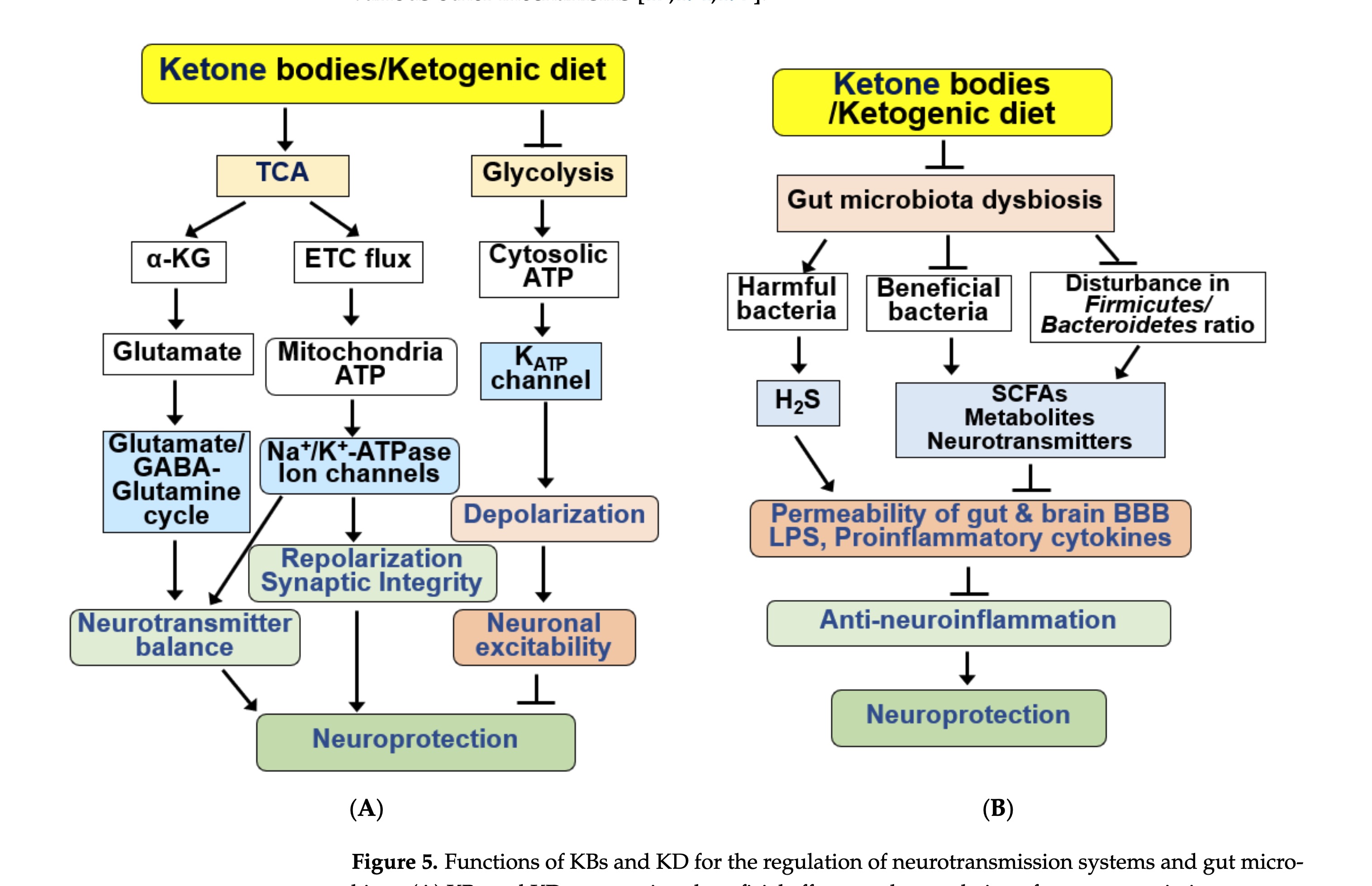
KD consumption has been shown to improve emotional symptoms, such as anxiety and depression, in patients with AD by modulating the glutamatergic neurotransmission system
The ameliorating effect of a KD on ASD-like behaviors is related to reduced levels of pro-inflammatory cytokines and microbiota remodeling.
Some studies have reported that KD has negative effects on the gut microbiota [37 ]. KD has been shown to reduce the overall diversity of the gut microbiome due to its low carbohydrate content. As microorganisms utilize fiber as their primary energy source, some portions of the microbial community cannot survive in KDs with low carbohydrate content. Signs of intestinal dysbiosis were observed in a group of AD rats and patients with MCI after KD consumption…As the neuroprotective effect of a KD exhibited through the intestinal microbial community is influenced by individual factors, such as sex, age, and race, additional studies are needed to identify the underlying mechanism
I wonder how the papers define dysbiosis, and if there is a net negative impact on overall health? Consider the diet of obligate carnivores, is their gut bacteria in dysbiosis or does it find a steady state?
A KD causes a variety of gastrointestinal discomforts, including nausea, vomiting, constipation, diarrhea, low appetite, weight loss, headache, hyperuricemia, and development of dyslipidemia
This is interesting, is this accounting for a diet readjustment period? i.e are these effects seen at 6 months? how many of these effects are due to a increase in plant based foods ?
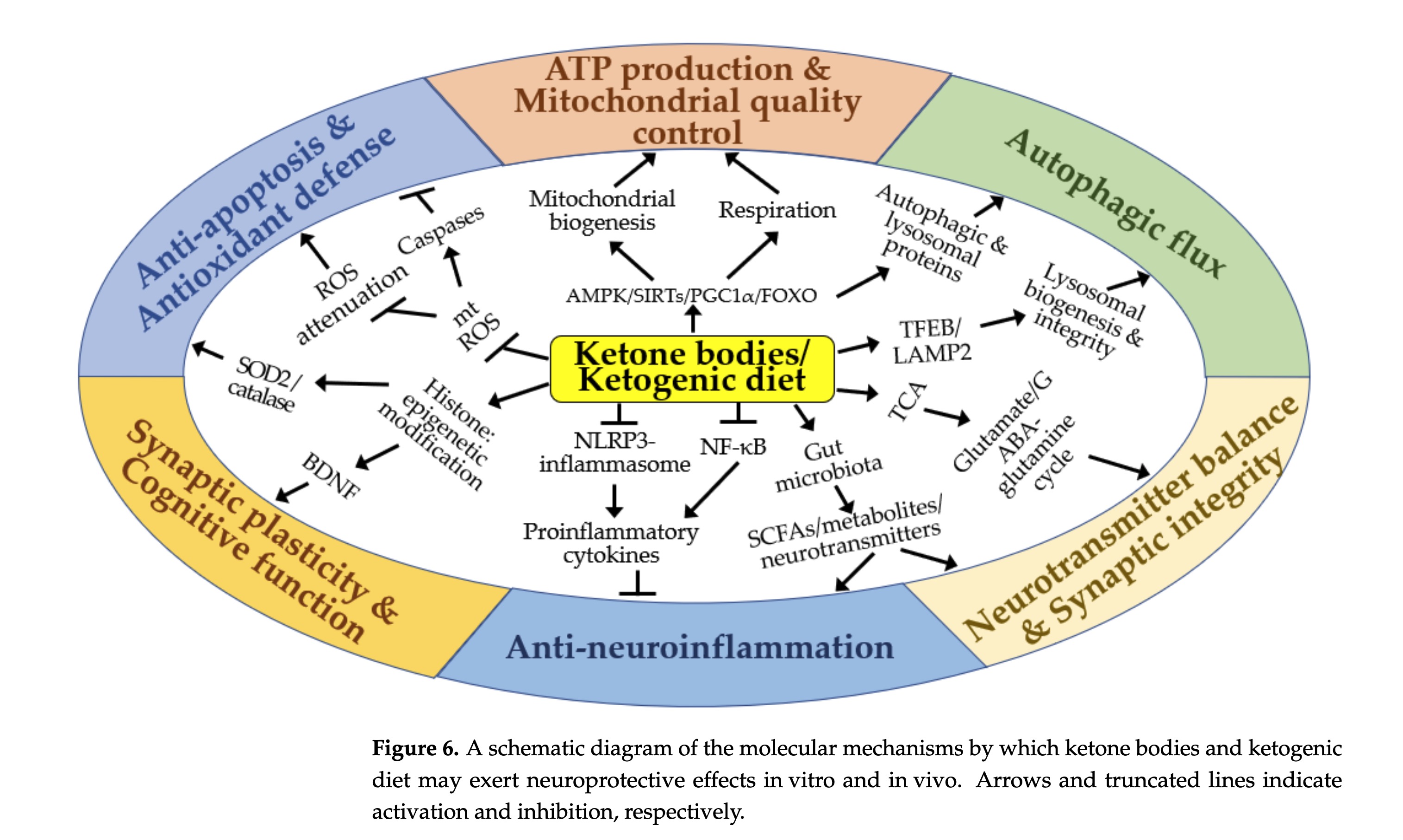
figure 6 - schematic of molecular mechanisms
.
I’m not sure why they are talking about ketoacidosis at all, is it relevant?
I think it’s because many people still, especially those working with diabetes, hear ketone and think of the uncontrolled diabetes crisis. I suppose it’s also vaguely useful to point out that an excess so very much in excess that it breaks the body’s ability to maintain correct acidity isn’t helpful. As if we don’t already know that everything is a poison, what matters is the dose.
That last bit about how poorly tolerated the keto diet is sounds very much like the first two weeks with poor guidance, or a badly designed ketogenic diet using foods some participants were intolerant to
I couldn’t do internet keto or Atkins keto for long. I didn’t tolerate it well. But zero carb is easy I have been doing it for 2 and a bit years.

